Up
(current research)
Cell
Deformability FPR, FCS,
FIDA Publications
Tissue equivalents reconstituted from isolated cells and defined matrix components have many advantages for studies of cell and tissue mechanics, interactions, and tissue development. One of these is simplicity relative to natural tissues. The initial proportion and type of cells and matrix constituents can be independently varied. Although cells secrete extracellular matrix (ECM) components during tissue development, these are relatively insignificant at early stages and may become more important later. This simplification of tissue composition permits a more informative characterization of the properties of the cells and matrix in the tissue and in comparison with the same constituents in isolation. Building up the complexity of composition of the tissue then can reveal the functions of the specific constituents added. For the same reasons observations of reconstituted tissues in their formative stages provides a useful and simple model of tissue development. Cell-cell and cell-matrix interactions are more readily observed than in the less controllable, more complex and crowded natural fetal tissues or wound models.
Reconstituted tissues also provide systems in which the physiological functions of selected cytoskeletal proteins can be determined. Using methods of molecular genetics, a cytoskeletal protein can be knocked out or its expression can be enhanced. Then the effect of this change on the mechanical characteristics of the tissue can be determined under conditions in which the contributions of the cells and of the matrix can be separately measured. In the long range another important consideration is the possibility of using reconstituted tissues clinically as replacements for injured or diseased natural tissues. An important task for the newly emerging field of Tissue Engineering is to develop methods for optimizing and characterizing the mechanical properties of reconstituted tissue targeted to medical or commercial application. The methods and approaches which we are developing will directly benefit this effort.
We have developed methods for measuring the force exerted by and
the viscoelasticity of connective, skeletal muscle and cardiac muscle
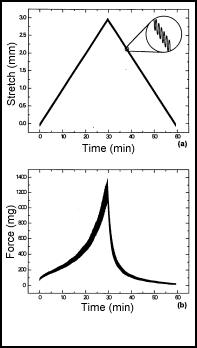
tissue equivalents (see papers cited below and Figure 1). 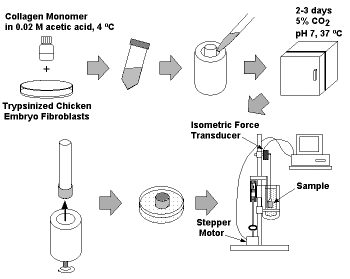 Using
these methods we are studying mechanical properties of cells and
matrix, mechanisms of tissue development, mechanisms of force
regulation, and functions of cytoskeletal proteins such as actin
depolymerizing factor and gelsolin. Figure 2 shows an example of cell
force exerted on a cardiac muscle tissue equivalent. As the
stretching force on the tissue is increased, the twitch force exerted
by the tissue increases up to the point at which the beat pattern
becomes irregular and erratic. Hence, the tissue equivalent mimics
the Frank-Starling behavior seen in authentic heart muscle.
Using
these methods we are studying mechanical properties of cells and
matrix, mechanisms of tissue development, mechanisms of force
regulation, and functions of cytoskeletal proteins such as actin
depolymerizing factor and gelsolin. Figure 2 shows an example of cell
force exerted on a cardiac muscle tissue equivalent. As the
stretching force on the tissue is increased, the twitch force exerted
by the tissue increases up to the point at which the beat pattern
becomes irregular and erratic. Hence, the tissue equivalent mimics
the Frank-Starling behavior seen in authentic heart muscle.